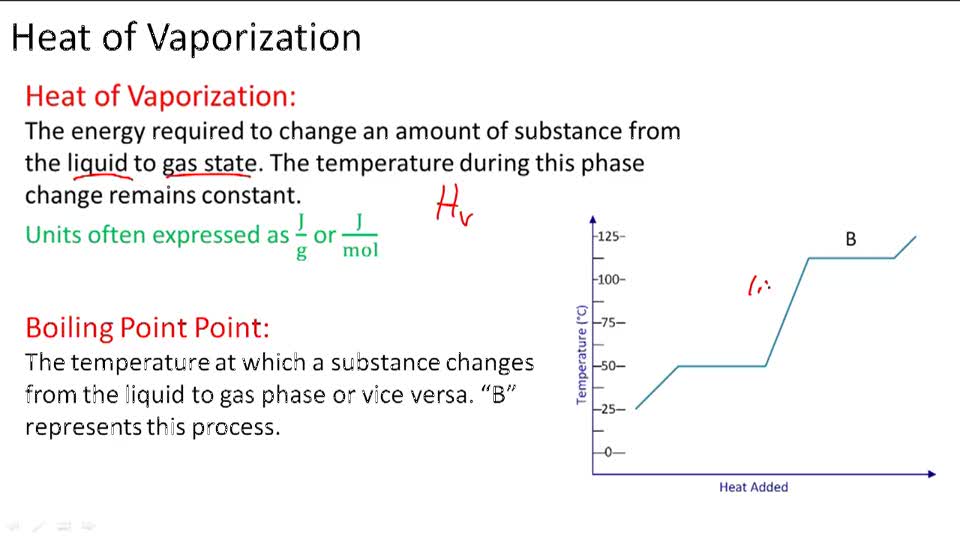
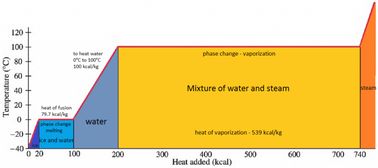
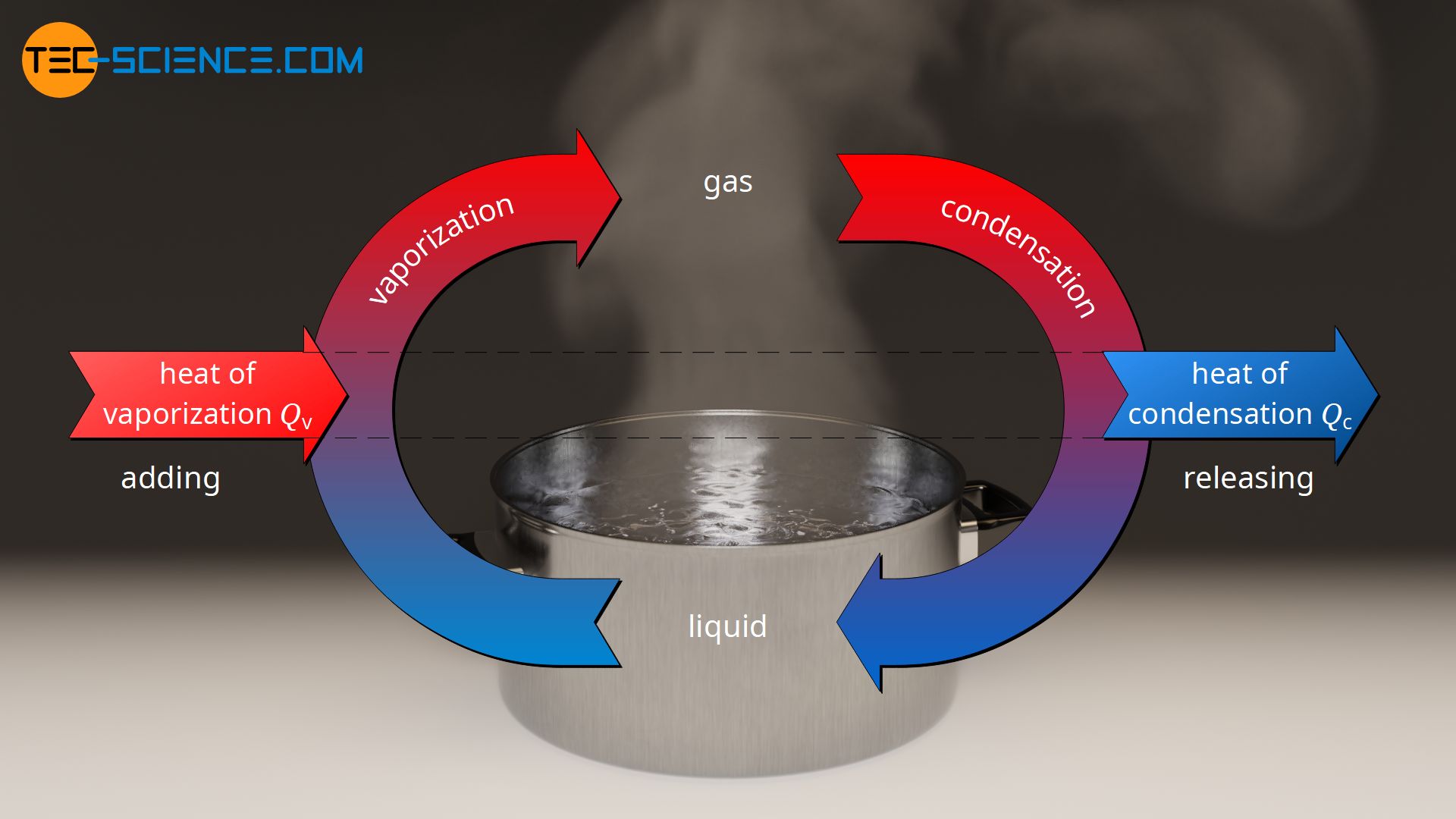
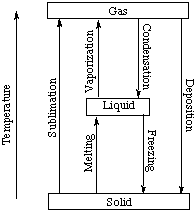
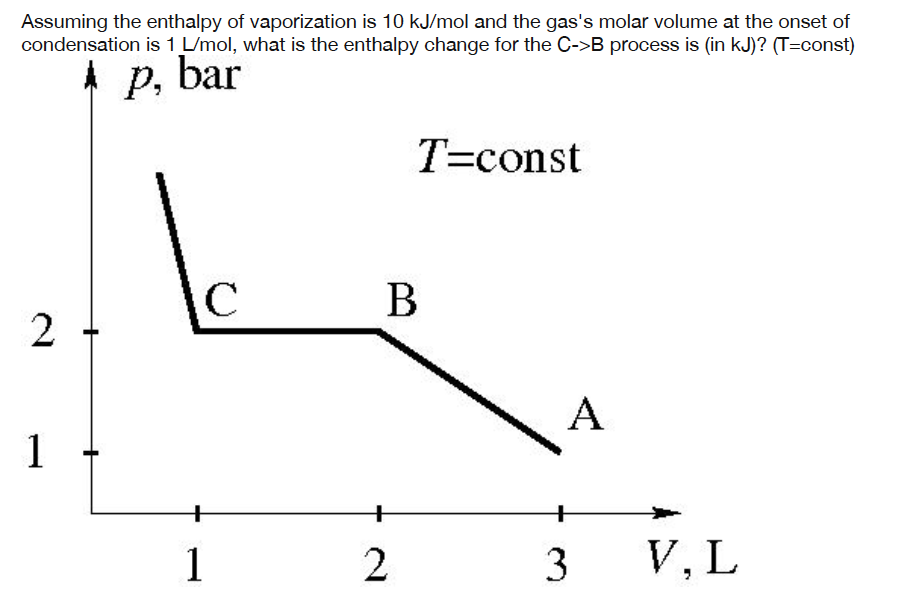
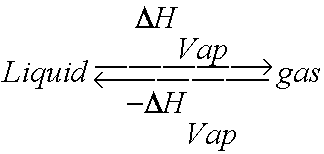Assuming the enthalpy of vaporization is 10 kJ/mol and the gas's molar volume at the onset of condensation is 1 L/mol, what is the enthalpy change for the C->B process is (in

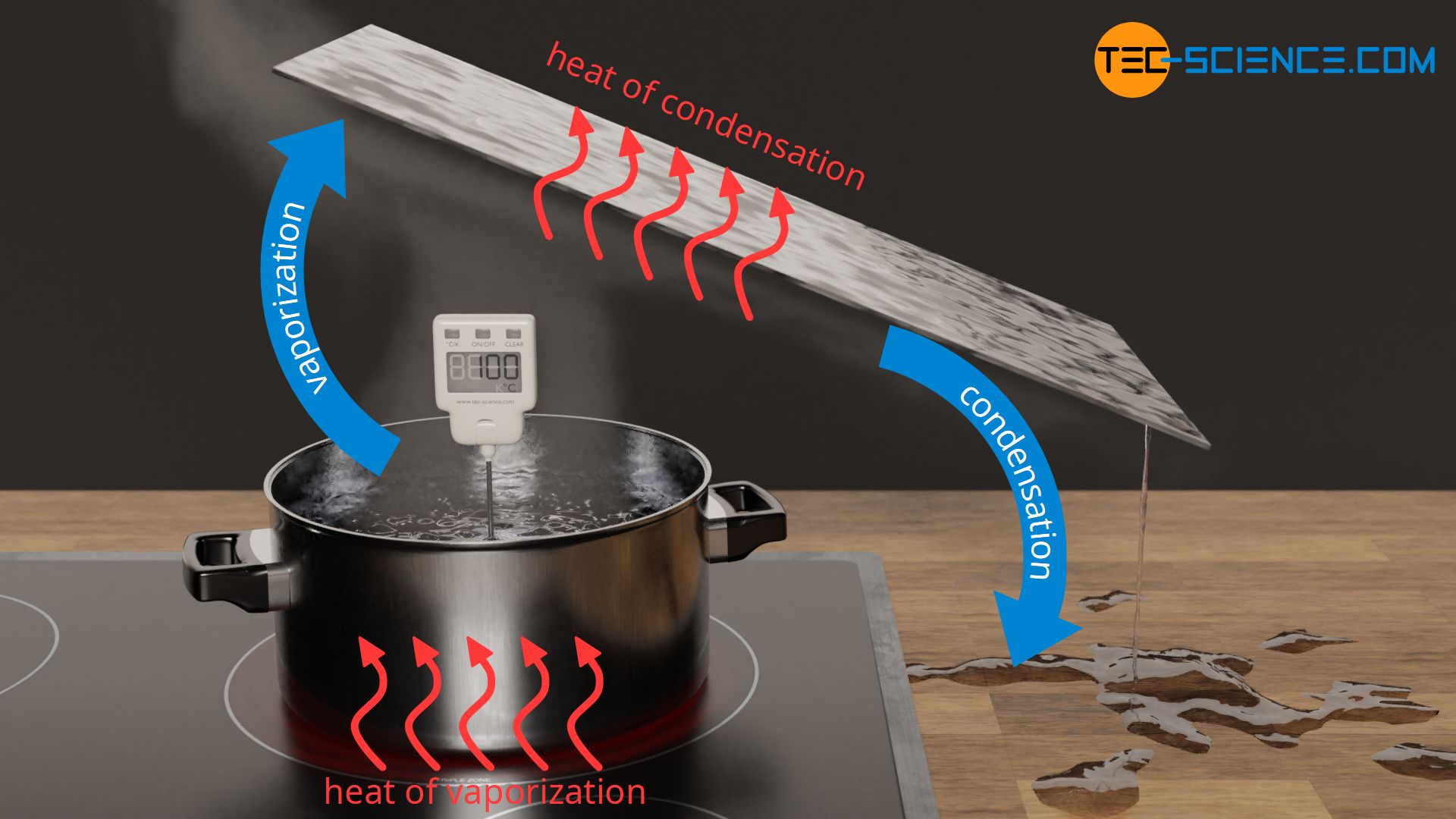
of the self-cooling and condensation effects on the molar enthalpies... | Download Scientific Diagram

Bomb Calorimetry constant volume often used for combustion reactions heat released by reaction is absorbed by calorimeter contents need heat capacity of. - ppt download
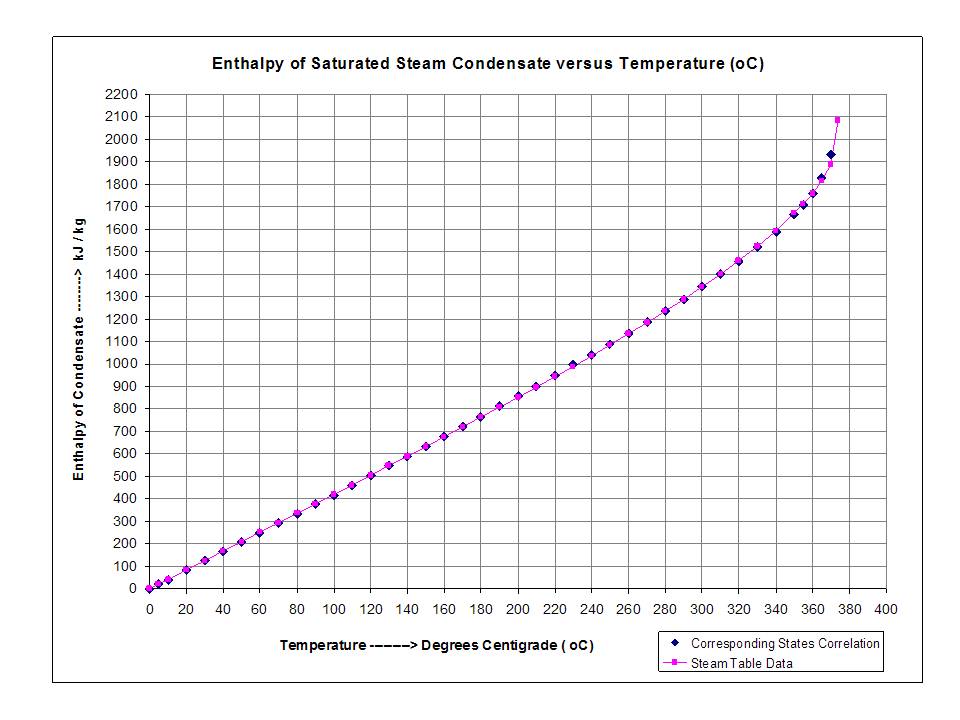
Calculation of Enthalpy and Density of Condensate ( saturated liquid Water) by corresponding states correlation | Chem-Eng-Musings

enthalpy - What is heat of vaporization? How can it be used at temperature as low as 25 °C? - Chemistry Stack Exchange